The clinical manifestation of Rett Syndrome is complex and quite heterogenous. Among the characteristic features of Rett Syndrome is a rather normal postnatal development for the first ~1.5 years of life, which is then followed by developmental stagnation, motor dysfunction, severe cognitive impairment, highly irregular respiration as well as epilepsy. At present, a cure for Rett Syndrome is not available.
In a mouse model of Rett Syndrome (Mecp2-knock out mice), we recently showed that mitochondrial changes already manifest in neonatal mice before the first typical disease symptoms occur. Also, by means of advanced optical redox sensors, we confirmed more oxidized redox conditions as well as a more vulnerable redox homeostasis in cultured hippocampal slices of male, MeCP2-deficient mice (Großer et al. 2012, Neurobiol. Dis. 48: 102-114).
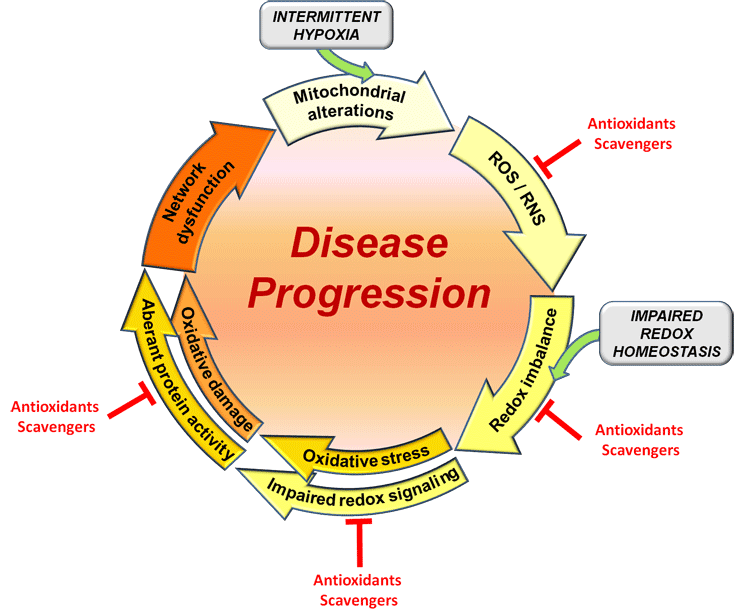
Based on these findings, we hypothesized that the early mitochondrial dysfunction and the associated cellular redox imbalance in Rett syndrome gives rise to subcellular molecular alterations, thereby contributing to disturbed neuronal responsiveness and neuronal network dysfunction and driving disease progression. In such a scenario, antioxidants and free-radical scavengers could mediate beneficial effects.
Indeed, we were able to confirm in isolated hippocampal tissue obtained from adult, symptomatic male Mecp2-knockout mice that the free-radical scavenger Trolox (a water soluble vitamin E derivative) dampens neuronal hyperexcitability and improves synaptic plasticity as well as the hippocampal hypoxia tolerance (Janc & Müller 2014, Front. Cell. Neurosci. 8: 56). These promising findings strongly support our working hypothesis.
Currently, we perform a detailed placebo-controlled blinded in vivo trial on MeCP2-deficient mice to rate in detail the potential pharmacotherapeutic merit of a chronic, systemic Trolox treatment.